Fumed Silica Technology
High Performance
Fumed Silica Technology
Completely closed manufacturing process;
Automatic combustion control system;
Single or multiple raw material mixing ratio;
Diversified product brands and high single - line production capacity; Application research and development laboratory.
The technology of this project is optimized and innovated on the basis of absorbing foreign advanced technology experience, so that the investment of the device is lower.
Compared with the domestic open process technology, the advantages are huge, and it can fully reach the technical level of foreign - funded enterprises:
A、High annual production capacity of a single line: ;;::5000 tons
(silicon tetrachloride as raw material);
B、The variety of reaction raw materials, STC, TCS, MTS can be mixed at any proportion to produce fumed silica;
C、Closed process manufacturing: from raw materials to products fully closed system, product impurities are very few;
D、Automatic control of manufacturing equipment, safe and convenient operation;
E、The operation and maintenance cost is low, and the maintenance is planned once a year;
F、The product brand is complete, from low-end products to high-end products to meet the needs of different fields,
G、Environmental protection and safety: harmless treatment of three wastes, clean and safe working environment.
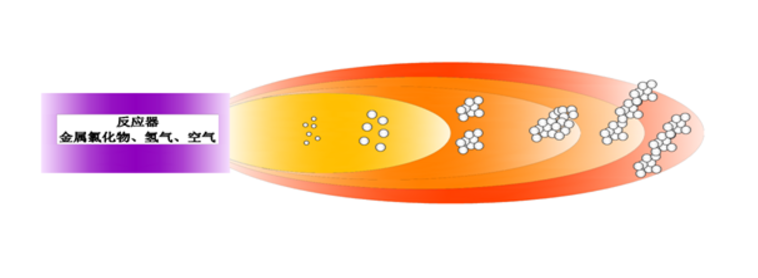
Raw Materials and Reaction Equation
The primary raw materials for the production of fumed silica are chlorosilanes, hydrogen, and air. The main types of chlorosilanes used in this process include silicon tetrachloride (SiCl₄), trichlorosilane (SiHCl₃), methyltrichlorosilane (CH₃SiCl₃), and dichloromethylsilane (CH₃HSiCl₂).
Silicon Tetrachloride (SiCl₄) : SiCl4+2H2+O2→SiO2+4HCl
Trichlorosilane (SiHCl₃) : SiHCl3+1.5H2+O2→SiO2+3HCl
Methyltrichlorosilane (CH₃SiCl₃) : CH3SiCl3+2H2+3O2→SiO2+3HCl+2H2O+CO2
Dichloromethylsilane (CH₃HSiCl₂) : CH3HSiCl2+H2+3O2→SiO2+2HCl+2H2O+CO2
Summary of the Reaction Process:
In all cases, the chlorosilane (SiCl₄, SiHCl₃, CH₃SiCl₃, or CH₃HSiCl₂) undergoes hydrolysis in the presence of hydrogen and oxygen at high temperatures (1500°C to 2000°C). The reactions produce fine silica particles (SiO₂), hydrogen chloride (HCl), and other byproducts such as water (H₂O) and carbon dioxide (CO₂) depending on the type of chlorosilane used. These reactions take place in a flame enviro In all cases, the chlorosilane (SiCl₄, SiHCl₃, CH₃SiCl₃, or CH₃HSiCl₂) undergoes hydrolysis in the presence of hydrogen and oxygen at high temperatures (1500°C to 2000°C). The reactions produce fine silica particles (SiO₂), hydrogen chloride (HCl), and other byproducts suc nment, leading to the formation of fumed silica, which is then collected and processed for use in various industrial applications.